Describe Jj Thomson Model of Atom
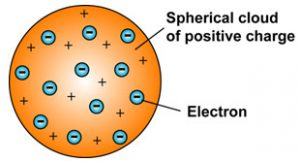
The atom in Thomsons model resembles a plum pudding. Because the negative and positive charges are of equal size an atom is electrically neutral as a whole.

Explain The Thomson S Model Of An Atom With A Neat Diagram Brainly In
Thomson discovered a negatively charged particle called electron which he assumed to be 2000 times lighter than a proton.

. Thomson proposed his model of the atom in 1903then only electrons and protons were known to be present in the atom. To explain the two. An atom consists of a positively charged sphere and the electrons are.
This model did not tell us about the presence of neutrons in the atom. Thomsons plum pudding model and E. Which one is the correct model and why.
Ad Over 27000 video lessons and other resources youre guaranteed to find what you need. Up to 24 cash back Description of his model. Because he observed that electrons were.
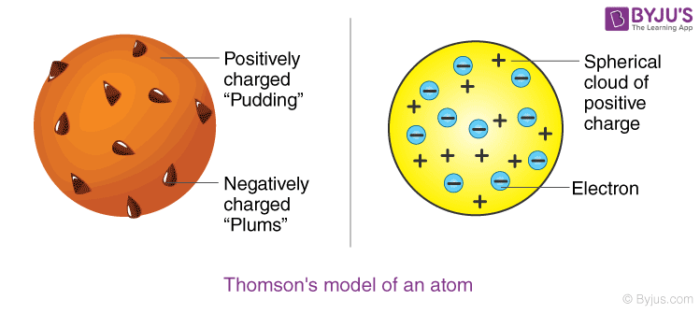
The JJ Thomsons plum pudding model establishes that when he researched the atom he found that electrons are positively charged. 03 Thomsons Model of The Atom In 1897 J. The plum pudding model.
The Plum Pudding Model is a model of atomic structure proposed by JJ. According to Thomson Model of an atom. Which one is the correct model and why.
So he proposed a model on the basis of known properties available at that time. Thomson in 1898 proposed that an atom possesses a spherical shape radius approximately 10 -10 m in which the positive charge is uniformly distributed. Thomson who invented the electron in the year 1897 suggested the atoms plum pudding model in 1904 which was for including the electron in the atomic model.
In 1897 Thomson claimed that the basic body of an atom is spherical in shape that contains electrons tiny particles within the atom that create a negative charge and a positively charged jelly around the electrons that neutralize the charge of the electrons. He stated that atoms consist of a positively charged sphere and electrons are embedded into it. I An atom consists of a positively charged sphere and the electrons are embedded in it.
It was proposed by JJ Thomson in the year 1904 just after the discovery of electrons. The electrons are embedded into it in such a manner as to give the most stable electrostatic arrangement Figure. The correct answer to this open question is the following.
In his atomic structure model he considered atoms to have a cloud. The description of Thomsons atomic model is one of the many scientific models of the atom. Rutherfords empty space atomic model of an atom.
Scattered in this fluid were negatively charged electrons these were the plums in the pudding. Ii The negative and positive charges are equal in magnitude. The positive and the negative charges in an atom are equal in magnitude due to which an atom as a whole is electrically neutral.
After discovering the electron in 1897 J J Thomson proposed that the atom looked like a plum pudding. Embedded in itThe negative and positive charges are equal in magnitude. However at that time the atomic nucleus was yet to be discovered.
So the atom as a whole is electrically neutral. Thomsons plum pudding model and E. Jj thomson proposed his model of atom in the year 1903according to him-An atom consists of a sphere of positive charge with negatively charged electrons embeded in itThe positive and negative.
So the atom as a whole is electrically neutralThomsons model of the atom fails to explain Rutherfords α-particle scattering experiment in which most of the fast moving α-particles. An atom consists of a sphere of positive charge with negatively charged electrons embedded in it. Rutherfords empty space atomic model of an atom.
2The positive and negative charges in an atom are equal in magnitudedue to which an. Thomsons model was known as the Plum Pudding Model or Raisin Bread Model As each atom was a sphere filled with a positively charged fluid known as the pudding. Thomson proposed the model of an atom be similar to that of a Christmas pudding.
The first postulate states that an atom is made up of a positively charged sphere with electrons contained within it. Watermelon is compared to the Thomson atomic model. Thomson in the late 19th century.
Thomson had discovered that atoms are composite objects made of pieces with positive and negative charge and that the negatively charged electrons within the atom were very small compared to the entire atom. JJ Thomsons Model of Atom Thomson proposed a theory according to which he defined atoms to be similar to that of a Christmas pudding. Thomson was the first one to propose a model for the structure of an atom.
Thomson Model of an atom. 1An atom consist of a sphere of positive charge with negatively charged electrons embedded in it. The electrons were assumed to be positioned in revolving circles around the atom in this model to be having a cloud of positive charge.
J J Thomson Model Of An Atom Class 9 Structure Of An Atom
Thomson Atomic Model Plum Pudding Model Postulates Limitations

Thomson Atomic Model Description Image Thomson Atomic Model Thomson Atom Atom
Comments
Post a Comment